Abstract
Background
Thromboembolism (TE) is a serious complication in pediatric patients with ALL associated with significant morbidity, mortality and therapy alterations. Thromboprophylaxis, though effective in preventing TE in patients at high risk for TE, is associated with increased risk of bleeding. Hence it is important to identify the population at the highest risk for TE for whom preventive strategies may be warranted. However, predictors of TE in pediatric patients with ALL remain uncertain and the role of inherited thrombophilia is ill defined. Adult studies of non-cancer patients and our prior smaller pilot study of pediatric ALL patients showed higher rates of TE with non-O blood types.
Aim
To identify variables at the time of ALL diagnosis that are associated with increased risk of TE in children and adolescents treated on DFCI ALL 05-001 trial.
Design/Methods
Eligible and consenting patients (1 to ≤18 yrs. of age) with newly diagnosedALL were enrolled on the DFCI 05-001 trial between 2005-2011 at 11 participating institutions in the USA and Canada. Risk categorization and protocol therapy have previously been described (Lancet Oncol 2015;16:1677-90). Treatment included one dose of pegaspargase (2500 IU/m2) during remission induction and 30 consecutive weeks of L-asparaginase during post-induction therapy (either pegaspargase 2500 IU/m2 every 2-weeks or native E.coli L-asparaginase 25000 IU/m2 weekly). Baseline clinical and laboratory data as well as all TE events requiring intervention (Grade 2 or higher) were prospectively collected. Genomic DNA was isolated from peripheral blood or bone marrow obtained at the time of complete remission in participants who consented for the collection of research samples. Single nucleotide polymorphisms (SNP) were detected using PCR-based allelic discrimination assays for prothrombin (PT)gene G20210A (rs1799963) and Factor V G1691A (rs6025) (Factor V Leiden, FVL) (Applied Biosystems, Carlsbad CA). The cumulative incidence of TE was estimated with induction failure, relapse and death considered as competing events. Effect of clinical [age, gender, body mass index (BMI) ALL immunophenotype, risk-group] and laboratory variables [presenting leukocyte count, blood group, SNPs for PT and FVL] at ALL diagnosis on the cumulative incidence of TE was evaluated in univariate and multivariable competing risk regression models. The impact of TE on ALL outcome (event free and overall survival) was further explored using a time-varying covariate in Cox regression modelling.
Results
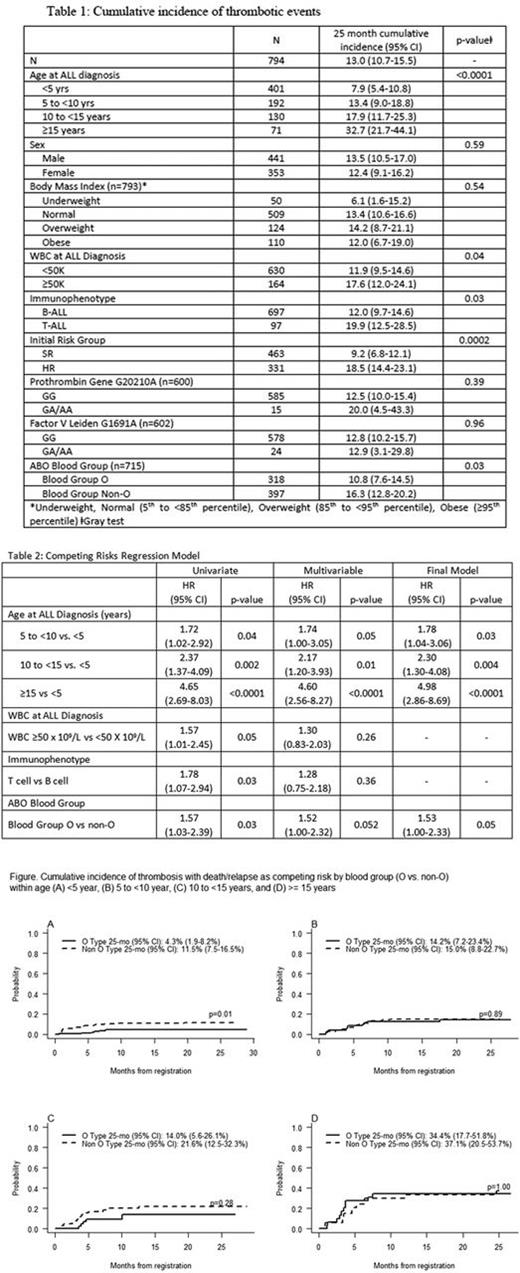
Of 794 enrolled patients [median age 4.97 (range 1.04 -17.96) yrs.; males 441], 100 developed TE; the 25-month cumulative incidence was 13.0% (95%CI 10.7,15.5). Twenty-one patients had 21 events during induction (3 with CNS TE) and 79 patients had 88 events post-induction (16 CNS TE). Table 1 provides the cumulative incidence of the time to first event of TE by patient characteristics and is compared using Gray test. Sex, BMI and thrombophilia SNPs had no detectable impact on the incidence of TE. There was no difference in incidence of TE by asparaginase type (data not shown). In the final competing risk multivariable regression model older age group and non-O blood group were each significantly associated with TE (Table 2). Figure 1 depicts the effect of blood group type on the incidence of TE within each age group; the cumulative incidence of TE was significantly higher for non-O blood group only for patients <5 years of age (p=0.01). There was no difference in the event free or overall survival between patients with or without TE.
Conclusion
Older age and non-O blood group are significant predictors of TE during ALL therapy. The effect of non-O blood group is more pronounced in younger age patients. Polymorphisms of FVL and PT gene had no impact on the risk of TE. We recommend further evaluation of these risk factors and recommend thromboprophylaxis for pediatric patients with ALL over 10 years of age (and especially those 15 years or older) receiving asparaginase-intensive regimens, and consideration of thromboprophylaxis in younger patients with non-O blood group.
Neuberg: Synta Pharmaceuticals: Other: Stock shares.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal